This page is part of the site called Surgical Pathology of the Canine Female Reproductive Tract by
University of Guelph
©All materials are copyright and should not be used without the expressed permission of the author
Table of Contents
|

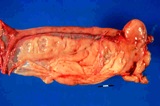
Normal reproductive tract of a dog with ovary shrouded in an ovarian bursa.
The main structures include theMcEntee (1991) provides a detailed review of the structure of the normal canine ovary.
Piseddu et al (2012) provides the cytological appearance of smears from normal ovaries with a histological correlate.
Piseddu E, Masserdotti C, Milesi C, Solano-Gallego L. (2012) Cytologic features of normal canine ovaries in different stages of estrus with histologic comparison. Vet Clin Pathol. 2012 41: 396-404.
Ovarian bursa
The bursa of the ovary is an extension of the mesovarium that almost completely surrounds the ovary in dogs. In cats it is only on the lateral surface of the ovary. In the dog, there is a small clear area on the lateral surface. This is described by McEntee (1991) as an area of the bursa without fat and this is on the lateral side. There is a small 2 x 15 mm slit or opening to the peritoneal cavity on the medial side.
A histological feature of the ovarian bursa and therefore the mesovarium is a thin discontinuous layer of smooth muscle beneath the mesothelial cells. This is helpful in orientation and in identifying the location of ovarian remnants.
Bursa surrounding the ovary. The opening to the peritoneum is a slit on the medial surface.
Surface epithelium
The epithelium of the ovary is a continuation of the mesothelium from the peritoneum. It is a layer of cells that varies from a simple squamous appearance to distinctly cuboidal or columnar. It therefore can have an epithelial appearance and this its name. It was called the germinal epithelium, and there are some that feel this is still appropriate as there is evidence that new oocytes can arise from this.
The immunostaining characteristics are indicated below.
Normal ovary with surface epithelium and subsurface epithelial structures in the capsule of the ovary.
Capsule of the ovary
This is the new name for the tunica albuginea of the ovary. It is a layer of dense fibrous tissue beneath the ovarian epithelium. It is more pronounced in older dogs, but is less cellular than the underlying ovarian stroma. It is the location for subsurface epithelial structures. The photomicrograph above show the capsule of the ovary.
Subsurface epithelial structures
The bitch is unique in having invaginations of the surface epithelium into the capsule of the ovary. These structures are called cortical tubules by Akihara et al (2007). Their characteristics are identical to the surface epithelium. They can become cystic, and are the most common site for the development of neoplasia. The photomicrograph above has 2 such structures. For the trivia buffs, they are also seen in marsupials, pinnepeds, elephants and primates (McEntee 1991).
Many believe that granulosa cells originate from these downgrowths embryologically (Stott 1974)
Akihara Y, Shimoyama Y, Kawasako K, Komine M, Hirayama K, Terasawa A, Ohmachi T, Matsuda K, Okamoto M, Taniyama H. (2007) Histological and Immunohistochemical Evaluation of Canine Ovary. Reproduction in Domestic Animals 42 (5): 495–501.
Ovarian stroma
The stroma of the ovary is composed of a unique connective tissue that is highly cellular and with little intercellular matrix. See the above photomicrograph.Granulosa cell rests
These structures, with the SES, are unique to the bitch. They are called 'endocrine cells' by Akihara et al (2007) but they are distinctly different to the interstitial endocrine cells that are occasionally seen and which are seen to surround the follicles (part of the theca interna). Stott (1974) suggests they are remnants of granulosa cells that remain after atresia of large follicles. McEntee (1991) appears to agree with this. They are also called granulosa cell islands and granulosa cell cords. They are seen in prepubertal bitches. Careful searching will reveal remnants of the zona pelucida within the lumen of some of these, and others will have hyalinised theca internal around them. They are more numerous in older bitches too. Stott (1974) also considered sex cord stromal tumors (granulosa cell tumors) to arise from these.
Granulosa cell rest (upper left) beside a developing follicle with central oocyte.
Stott GG (1974) Granulosa cell islands in the canine ovary; histogenesis histomorphologic features and fate. Amer J Vet Res 1974 35: 1351-1355
Follicles
Follicles begin as oocytes embedded in ovarian stroma - these are primordial follicles. Primordial follicles can have a single layer of squamous cells around them. Primordial follicles develop into primary follicles, where the single layer of squamous cells become a larger and cuboidal to columnar layer of granulosa cells. Granulosa cells produce cytokines that recruit theca cells from the ovarian stroma cells (Young and McNeilly 2010). Secondary follicles have oocytes surrounded by multiple layers of cells including granulosa cells, but there is no cavity or antrum. Tertiary follicles or vesicular follicles have an antrum.
To accurately calculate the size of a normal follicle, one must accurately measure the follicles of a large number of bitches, and determine the mean and standard deviation. Before the advent of ultrasonography, this would require ovarectomising bitches during estrus. McEntee (1990) reports the normal size of follicles in bitches as 5 to 8 mm. This is based on the work of Concannon et al (1977). In that paper, the dogs examined were beagles, and 5 were determined to have normal follicles, and they were between 5 and 8 mm in diameter. England and Allen (1989) reported that follicles in nine bitches (breed not specified) were between 7 and 11 mm in diameter based on ultrasound and subsequent macroscopic examination. Reynaud et al (2009) examined 24 beagle ovaries and 9 mixed breed dog ovaries. Follicles were up to 8 mm diameter. Reynaud et al 2005 examined the ovaries of 50 bitches, 22 beagles and 28 of various other breeds and found preovulator follicles to be between 5-7 mm. Songsasen et al (2009) examined 14 pairs of ovaries from bitches at ovariohysterectomy. Antral follicles (10 examples) were an average of 5mm with a range of 3 to 6.7mm in diameter. Based on these various publications, 8 mm seems like the maximum diameter of the normal canine follicle before ovulation.
Corpora lutea have anechoic centres that were smaller in metestrus. Luteinisation begins at ovulation and fills in the cavity from the periphery.
Normal primordial follicles within ovarian stroma. Also note the ovarian epithelium, capsule of ovary, and subsurface epithelial structures.
Secondary follicle (centre) with central oocyte and surrounding layer of granulosa cells - but no antrum.
Primordial (left upper) , primary (left upper), and early tertiary follicles (central right) in a dog. The edge of a large tertiary follicle at the lower edge.
Wall of tertiary follicle with granulosa cell layer and thecal layer below.
Concannon P, Hansel W, McEntee K (1977) Changes in LH, Progesterone and sexual behavior associated with preovulatory luteinization in the bitch. Biol Reproduct 1977, 17: 604-613.
England GCW, Allen WE. Ultrasonographic and histological appearance of the canine ovary. Vet Rec 1989 125: 555-556
K. Reynaud, C. Viaris de Lesegno, M. Chebrout, S. Thoumire, S. Chastant-Maillard. Follicle population, cumulus mucification, and oocyte chromatin
configuration during the periovulatory period in the female dog. Theriogenology 2009 72: 1120-1131K Reynaud, A Fontbonne1, N Marseloo1, S Thoumire1, M Chebrout, C Viaris de Lesegno1 Chastant-Maillard. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005 130: 193-201
Songsasen N, Fickes A, Pukazhenthi BS, Wildt DE. Follicular morphology, oocyte diameter and localisation of fibroblast growth factors in the domestic dog ovary. Reprod Domest Anim 2009 44 Suppl 2: 65-70.
Young JM, McNeilly AS. (2010) Theca: the forgotten cell of the ovarian follicle. Reproduction 2010 140: 489-504
Rete ovarii
The rete ovarii are tubules that occur in the medulla at the hilus of the ovary. There are 3 components - the intraovarian rete, the connecting rete, and the extraovarian rete. The latter 2 are ciliated and the former is not.
Akihara et al (2007) described 2 histological patterns in the canine ovary - lower cuboidal epithelium with reticular growth and
The rete ovarii of the mouse has been studied in detail (Byskov 1978). Early in embryonic development, all of the extraovarian rete begin as mesonephric tubules. The cranial 3 of 5 join the mesonephric duct, but the caudal ones regress and loose their connections. Close to the ovary, the tubules become cell cords. These cords branch and reconnect to form the connecting rete. Several cords penetrate into the ovarian stroma to form the intraovarian rete. Cords of coelomic epithelium enter some ovary from the surface and connect with the intraovarian rete. These cell cords from the coelomic surface are the destination for the germ cells and the future development of the follicles. In other species there is such a close association between intraovarian rete and germ cells.
columnar epithelium with tubular growth.Akihara Y, Shimoyama Y, Kawasako K, Komine M, Hirayama K, Terasawa A, Ohmachi T, Matsuda K, Okamoto M, Taniyama H. (2007) Histological and Immunohistochemical Evaluation of Canine Ovary. Reproduction in Domestic Animals 42 (5): 495–501.
Byskov AG. (1978) The Anatomy and Ultrastructure of the Rete System in the Fetal Mouse Ovary. Biology of Reproduction 1978 19 (4): 720-735
Primary and secondary follicles produce alpha inhibin. This inhibits the action of activin, which has a proliferative effect on granulosa cells and which assists in the maturation of the oocyte. Inhibin does not appear to be produced by granulosa cells during the latter stages of follicular development.
AntiMullerian hormone is produced in low concentrations in normal ovaries. Walter et al (2018) reported normal serum AMH to be 0.12 to 0.99 ng/ml.
Marino G Zanghı A (2013) Activins and Inhibins: Expression and Role in Normal and Pathological Canine Reproductive Organs: A review. Anatomia Histologia Embryologia 2013,
Walter B, Coelfen A, Jäger K, Reese S, Meyer-Lindenberg A, Aupperle-Lellbach H. Anti-Muellerian hormone concentration in bitches with histopathologically diagnosed ovarian tumours and cysts. Reprod Domest Anim. 2018 Jun;53(3):784-792.
Ricchardi et al (2007) reported on the staining characteristics of 4 normal ovaries, 8 granulosa cell tumours and 6 epithelial tumours using cytokeratin 7, cytokeratin AE1/AE3, vimentin and inhibin alpha. This is the general staining pattern. The normal ovarian surface epithelium was cytokeratin and vimentin +, and granulosa cells had variable staining for all except CK7 which was uniformly negative and inhibin alpha which was uniformaly +.
AE1/AE3 CK7 Vimentin Inhibin alpha
Akihara et al (2007) performed a histological and immunohistological study of 15 normal canine ovaries. The normal structures they identified, and their staining characteristics are as follows
surface epithelium subsurface epithelial structures SES
tunica albuginea stromal cells granular cell rests rete ovarii uterine tube
AE1AE3 CK7 CK13 CK20 Vimentin Desmin SMA Calponin S100 NF inhibin alpha NSE Placental alk phos
Y Akihara, Y Shimoyama, K Kawasako, M Komine, K Hirayama, A Terasawa, T Ohmachi, K Matsuda, M Okamoto, H Taniyama (2007) Histological and Immunohistochemical Evaluation of Canine Ovary Reproduction in Domestic Animals 42 (5): 495–501.
English GCW, Allen WE (1989). Ultrasonographic and histological appearance of the canine ovary. Vet Rec 125: 555-556.
Hoffmann B, Büsges F, Engel E, Kowalewski MP, Papa P.(2004) Regulation of corpus luteum-function in the bitch. Reprod Domest Anim. 2004 Aug;39(4):232-40.
Riccardi E, Greco V, Verganti S, Finazzi M. (2007) Immunohistochemical diagnosis of canine ovarian epithelial and granulosa cell tumors. J Vet Diagn Invest 19: 431-435
The normal canine uterus is a bicorniate structure composed of 2 horns and a body.
Endometrium
Bartel et al (2014) studied the lipid laden surface endometrial cells of the uterus in metestrus/diestrus using electron microscopy and immunohistochemistry. These cells are normal and are considered part of pregnancy/pseudopregnancy of bitches.
Bartel C, A. Tichy A, Walter I (2014) Characterization of Foamy Epithelial Surface Cells in the Canine Endometrium. Anat Histol Embryol 2014; 43: 165-818
Cytokine profile
Payan-Carreira et al (2011) reported on the immunohistochemical localisation of TNF in the endometrium of normal bitches during the estrus cycle. Staining was found in the contents of glands in anestrus and proestrus, within the fibroblasts especially and also the epithelium. It was seen in the endothelium of capillaries. During pregnancy, it is present in foetal tissues, trophoblasts and endometrium but not surface endometrial epithelium.
Payan-Carreira R, Pires MA, Ström Holst B, Rodriguez-Martinez H. (2011) Tumour Necrosis Factor in the Canine Endometrium: An Immunohistochemical Study Reprod Dom Animal 2011 46: 410–418.
Normal lymphocytes
Bartoskova et al (2012) examined normal uteri of dogs, identifying B cells (CD21), T helper cells CD3, CD4, Cytotoxic T cells (CD3 CD8) and gamma delta T cells (gamma delta T cell receptor).
Pires et al (2015) examined the endometria of 50 pubertal dogs and 5 were in the post partum period. There are normal resident cells at all stages of the cycle. They were most prominent in the late involution period. Macrophages were most common in beneath the lumenal epithelium in anestrus.
Bartoskova A, Turanek-Knotigova P, Matiasovic J, Oreskovic Z, Vicenova M, Stepanova H, Ondrackova P, Vitasek R, Leva L, Moore PF, Faldyna M. (2012) Gamma delta T lymphocytes are recruited into the inflamed uterus of bitches suffering from pyometra. The Vet J 2012 194: 303-309
Pires MA, Payan-Carreira R. Resident Macrophages and Lymphocytes in the Canine Endometrium. Reprod Domest Anim 2015; 50: 740-749.
ImmunohistochemistryThe endometrial epithelial cells stain with Pancytokeratin and CK7. It is CD10 negative
The stroma stains with vimentin, CD10 and often smooth muscle actin.
The smooth muslce cells of the myometrium is desmin and smooth muscle actin positive, vimentin negative and CD10 negative.
CD10 (Neprilysin, also known as membrane metallo-endopeptidase MME, neutral endopeptidase NEP) stains the endometrial stroma and myometrium. It is an enzyme that breaks down oxytocin, endothelins, and interleukin 1, so it helps regulate endocrine control of the endometrium.Trophoblasts are weakly CD10 positive
Additionally Human endometrial stroma is known to have smooth muscle actin and desmin positive cells.
Bartel C, Berghold P, Walter I. Ectopic endometrial tissue in mesonephric duct remnants in bitches. Reprod Domest Anim. 2011; 46: 950-956.
Czernobilsky B, Gabbiani G, Prus D, Lifschitz-Mercer B. Alpha-smooth muscle actin-positive myofibroblasts in endometrial stroma are not a reliable criterion for the diagnosis of well differentiated endometrioid adenocarcinoma in small tissue samples. Int J Gynecol Pathol. 2001 Jul;20(3):232-8. doi: 10.1097/00004347-200107000-00005. PMID: 11444198.
Czernobilsky B, Remadi S, Gabbiani G. Alpha-smooth muscle actin and other stromal markers in endometrial mucosa. Virchows Arch A Pathol Anat Histopathol. 1993;422(4):313-7. doi: 10.1007/BF01608341. PMID: 8506625.
Franquemont DW, Frierson HF Jr, Mills SE. An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am J Surg Pathol. 1991 Sep;15(9):861-70.
Payan-Carreira R, Santos C, Miranda S, Pereira RM, Santos D, Pires MA. Temporal changes in neutral endopeptidase/CD10 immunoexpression in the cyclic and early pregnant canine endometrium. Theriogenology. 2014; 82: 815-826.
Normal vaginal cytology has been described by many authors including Roszel (1977), Feldman and Nelson (2004),
Vaginal cytology is not always accurate (Fowler et al 1971), but is in the majority of cases.
Feldman EC, Nelson RW (2004) Ovarian cycle and vaginal cytology In. Canine and Feline Endocrinology and Reproduction. 3rd ed, Ch19 p 752-774. Saunders
Fowler EH, Feldman MK, Loeb WF (1971) Comparison of histologic features of ovarian and uterine tissues with vaginal smears of the bitch. Am J Vet Res 32: 327-334.
Roszel JF (1977) Normal canine vaginal cytology. Symposium on Reproductive problems Veterinary Clinics of North America 7: 667-681.
Allen and Dagnall (1982) cultured the vagina of 143 female dogs , 74 of which were in estrus, 47 were infertile or discharging and 22 were from kennels with infertility. The most frequent isolate was Escherichia coli, followed by Streptococcus, Staphylococcus and myriad other bacteria.
Baba et al (1983) examined the normal flora of the vagina and uterus including vaginal samples from 82 dogs and uterine samples from 78 samples. 79 of the 82 vaginal samples has bacteria. Bacteroides were cultured from 55%, Streptococci were cultured from 52%, Pasteurella was isolated from 34% and mycoplasma was isolated from 43%. 48 ot 72 uterine samples contained bacteria. Staphylococcus and Mycoplasma was the most frequent isolates.
Maksimovic et al (2012) cultured the vagina and uterus from 33 bitches at different stages of the estrus cycle and found a variety of bacteria including Staphlococcus, Streptococcus, E coli, Proteus, Corynebacterium and others. Pure cultures were found during proestrus and anestrus, but mixed cultures were found during diestrus and estrus. Some bacteria could be found in the uterus and most frequently during diestrus. The species were often different to those in the vagina.
Hutchins et al (2014) examined the vaginal flora of 44 spayed dogs, 21 of which had urinary tract infection. E coli (11/44) and Staphylococcus pseudointermedius (13/44) were the most common. There was no difference between those with urinary tract infections and normal.
Maksimovic et al (2018) cultured the vagina and uterus of clinically normal dogs for Mycoplasmas. Mycoplasma canis was the most common. 34% of dogs had Mycoplasma in their vagina and this did not change with the estrus cycle.
Teslin et al (2024) found Staphylococcus pseudintermedius (20%) and beta-hemolytic streptococci (18.33%) as the most common in pregnant dogs.
Allen WE, Dagnall GJR (1982) Some observations on the aerobic bacterial flora of the genital tract of the dog and bitch. J Small Anim Pract 23: 325-335.
Baba E, Hata H, Fukata T, Arakawa A (1983). Vaginal and uterine microflora of adult dogs.
Hutchins RG, Vaden SL, Jacob ME, Harris TL, Bowles KD, Wood MW, Bailey CS (2014) Vaginal Microbiota of Spayed Dogs with or without Recurrent Urinary Tract Infections. J Vet Internal Med 2014 28: 300-304
Maksimovic´A , Z. Maksimovic Z, S. Filipovic S, H. Beširovic H, Rifatbegovic M (2012) Vaginal and uterine bacteria of healthy bitches during different stages of their reproductive cycle. Vet Rec 2012, 171: 375-376
Maksimović Z, Maksimović A, Halilbašić A, Rifatbegović M. Genital mycoplasmas of healthy bitches. J Vet Diagn Invest. 2018; 30: 651-653.
Tešin N, Stančić I, Tekić D, Ačanski A, Kovačević Z. Prevalence and antimicrobial resistance trends among vaginal bacteria isolates from pregnant bitches. Reprod Domest Anim. 2024; 59: e14699.