Take precautions
It does not take long after reading the list of potential infectious causes of pregnancy failure to realise that the major infectious agents are potentially zoonotic. Of the top four causes, Coxiella burnetti is the most infectious and is well-known for causing community outbreaks. The spores of this agent are highly infectious and are spread by aerosol. They are long-lived and present in contaminated surroundings including dust in barns. sterilisation or rendering of spores in active is extremely difficult. Of the other agents, Campylobacter is spread by feco-oral transmission and Chlamydia is spread a variety of ways also. The precautions taken should reflect the potential risk. Even a closed flock that is known to be negative for Coxiella could have a breakdown in infection control. Diagnostic laboratories who handle potentially infectious material on a regular basis have standard operating procedures that include the use of special biohazard chambers, and the use of personal protective equipment that not only includes coveralls and gloves, but the special respirators and masks that filter ultrafine particles. An N95 mask with appropriate and verified fit testing is routinely used. In a field situation, use of suitable personal protective equipment including a system with a respiratory filter is highly recommended, particularly in those locations where vaccination for Coxiella is unavailable. Because many of the infectious agents are particularly hazardous in pregnancy, those individuals who are pregnant and who are in an altered immune competent state including the very young and the old are strongly advised to have no potential contact with these infectious agents. Appropriate disinfection, sanitisation and personal hygiene is a very important component to minimising risk of infection. Obviously the same recommendations hold for producers, farmers and their families or visitors.
Selection of material to examine
The general recommendations for selection of material and the numbers of cases to examine vary with the particular situation including the economic benefit. For individual cases, it is best to submit all placental and fetal tissues to an appropriate diagnostic laboratory for evaluation. It is best to provide the diagnostic laboratory with as much suitable and relevant information as possible, and to provide a maximum dollar figure for the investigation.Tentative diagnoses can be made by examining the placenta and fetus and will be described below. If there is an outbreak, a so-called abortion 'storm', a representative sample should be examined and if appropriate, submitted to the laboratory. At the field level, as many placentas and fetuses as possible should be examined and representative samples submitted for further diagnostics, if appropriate.
It is critical to submit the whole placenta, or at least a sample of a placenta that contains cotyledon and intercotyledonary placenta, and particularly any lesions.
The best samples to send to a diagnostic laboratory are whole placentas and foetuses. If their transportation is not possible, selection of samples should be done in accordance with the recommendations of the diagnostic laboratory. All diagnostic laboratories provide basic information on the samples they require and these instructions should be followed to the letter. A typical general submission requires a large number of samples that are appropriately taken, labelled, and packaged.
Typical containers appropriately labelled as per Users Guide of Animal Health Laboratory, University of Guelph.
Examination of Ewe(s)
Some infectious diseases are not easily confirmed - either because the agent is is very difficult to culture or identify, affects the fetus earlier in gestation and is no longer present, is neutralised by antibodies or myriad other causes. Serology may be the most accurate way of identifying the influence of an infectious agent. Collecting serum samples from a representative sample of the flock including serum from ewes with failure of pregnancy, non-pregnant and unaffected pregnant ewes should provide additional confirming evidence. It is best to check with the diagnostic laboratory if this option is available.
It is rare for reproductive samples to be taken from ewes however as a general principle, sampling of endometrial fluids may be an alternative to sampling a placenta if the placenta is no longer available
Examination of lamb(s)
Examination of a lamb or lambs is a much more involved process of examining a ruminant placenta.a thorough examination involves making observations that are different to those normally made at necropsy. In particular, recording the approximate age or length of gestation at the failure of pregnancy, identifying Fetal autolysis, and selectively examining particular tissues to achieve maximum diagnostic potential are required.
Age of lamb at loss
Most of the failures of pregnancy in sheep occurs late in gestation, and many people do not measure specific information on the degree of fetal development. Richardson et al (1976) provides information of the aging of fetal lambs. The classic measure is crown - rump length (they called it crown to anus length). This was accurate between 50 and 100 days of gestation. it is also worthwhile noting degree of wool cover and the type of coat. These measures should be written on any submission sheets and they form part of the record of your investigation
Time since death en utero
Many people are unaware of the normal changes of foetal autolysis and much of the older and even some of the newer literature lists autolytic changes as part of the spectrum of lesions of disease. Although this is not a critical observation in sheep, it is still worthwhile, conceptually, to determine if the fetus initiated its own birth (no autolysis) or whether it died rapidly en utero and became autolysed. In general, autolysis in stillborns is minimal, but autolysis in foetuses progresses rapidly because they are held at body temperature. Fetal autolysis is very different to autolysis of adult animals because they are in a sterile environment and do not have any intestinal flora.
Dillman and Dennis (1976) provided a detailed account of the autolytic changes that occur in ovine foetuses. A brief synopsis of their findings is provided in the following table.
Time since death
Change
12 hours
cornea cloudy, amnionic fluid blood tinged
24 hours
fluid in body cavities
36 hours
gelatinous fluid in subcutis
72 hours
Eyes dehydrated
144 hours
carcase dehydrated, no abomasal contents
Dillman RC, Dennis SM. Sequential sterile autolysis in the ovine fetus: macroscopic changes. Am J Vet Res. 1976; 37: 403-407.
Collection of samples
The post mortem examination of the fetus is very similar to that of an adult, at least in the initial phases. All necropsy is should be "problem based" and in the case of a fetal necropsy the aim is to identify those lesions that are significant to failure of pregnancy and to concentrate on those organs and tissues which are likely to have lesions. The following is a list of organs that should be sampled in every case – some of these are rarely sampled in an adult. A list of samples to take is usually provided by the diagnostic laboratory and to ensure all samples are taken the list of samples can be written on a paper plate and as the necropsy proceeds, pieces of tissue can be placed on the appropriate sample name on the plate.
Organs to be sampled in every fetus, in addition to routine samples from all major organs:
- Brain
- Thyroid gland
- Abomasal content
- Femur
Dillman RC, Dennis SM (1976) Sequential sterile autolysis in the ovine fetus: macroscopic changes. Amer J Vet Res 1976, 37: 403-407.
Examination of placenta(s)
Measurement of placental and fetal weight is a routine procedure for many species. Fetal weight is well known to be related to perinatal mortality with a birth weight above 5kg being a major risk factor for dystocia, stillbirth and subsequent mortality in the first hours of life.
Placental weight is seldom measured in sheep, except in a research setting. It is known that placenta weight increases above normal in midgestation (between 30 and 96 days) if there is maternal undernutrition. This is presumably a mechanism to compensate as fetal growth is not affected by undernutrition during this stage (McCrabb et al 1991).
Placental lesions in infectious disease can contain huge numbers of highly infectious particles and it is essential to take precautions (see above).
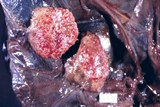
Lesions fall into two main types - cotyledonary necrosis and intercotyledonary placentitis. The lesions of focal cotyledonary necrosis are subtle and are often missed. Placentitis is usually very obvious.
Focal cotyledonary necrosis is the hallmark lesion of Toxoplasmosis. All cotyledons are usually affected and in most cases about 50% of the cotyledonary area. There are 1or 2mm white foci throughout the cotyledons.
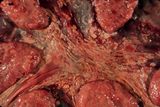
Placentitis comes in 2 forms with the chronic form being the most common. The acute form is more difficult to identify. The classical chronic changes are
- thickening and opacity of the intercotyledonary membranes
- fibrosis of the intercotyledonary tissues
- edema of the intercotyledonary tissues
- exudate overlying the intercotyledonary region
- cupping of the cotyledon
- reduced distance between the cotyledons
- regional cotyledonary necrosis
The typical acute lesions are subtle:
- exudate in the intercotyledonary placenta
- reddening of the intercotyledonary placenta
- edema of the placental tissues
- infarcts of the cotyledons